Managing Asthma with Controller Medications
Asthma is a major health concern in the United States due to poor disease control.
Asthma is a major health concern in the United States due to poor disease control. An estimated 23 million Americans have asthma, of whom 12 million experience an asthma exacerbation annually.1Many patients with asthma are not being prescribed or actively taking a long-term controller medication, even though they are needed to manage asthma effectively and prevent exacerbations. Promoting the use of long-term controller medications as part of a patient’s management plan is key to managing this disease.
Current Shortfalls in Asthma Control
Lack of health care access, insufficient education, medication side effects, poor provider management, and lack of patient follow-up are the main reasons for the inadequate use of long-term controller medications. Lack of asthma control leads to an increased number of asthma exacerbations, which account for an estimated 1.8 million emergency room visits each year in the United States.2Astonishing study results suggest that only 35.4% of the current asthma population uses a long-term controller for prevention of asthma exacerbations.1In order to decrease the occurrence of asthma exacerbations and improve disease control, practitioners should become more familiar with long-term controller medications.
Long-Term Controller Medications for Asthma Management
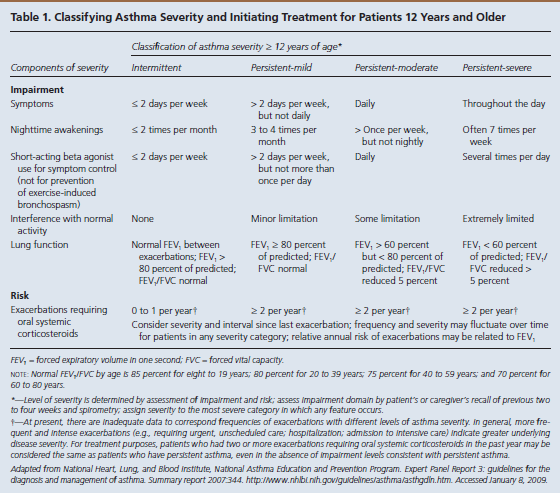
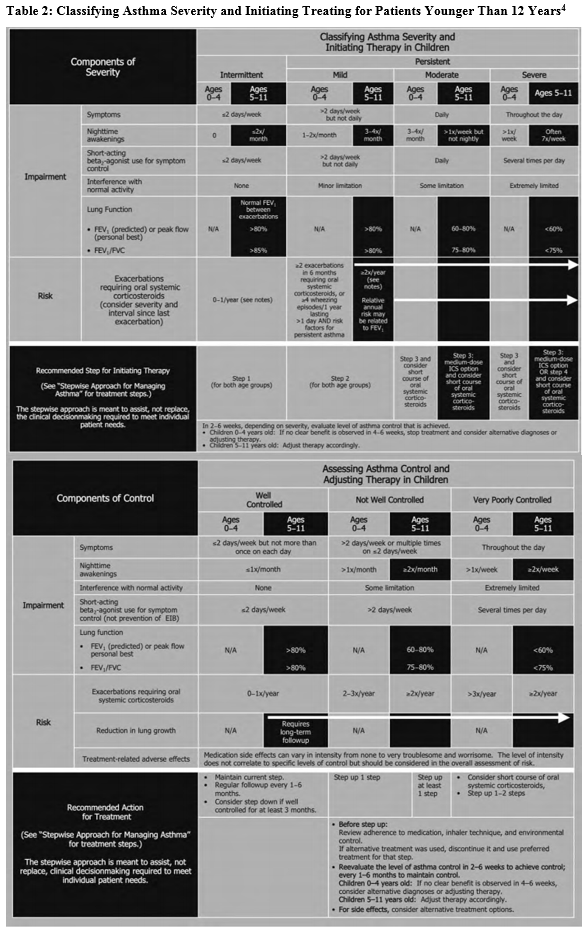
The goal of asthma disease management is to control the patient’s asthma in order to decrease the number of exacerbations. Guides are available to assist practitioners in classifying asthma severity in patients aged 12 years and older (Table 13) and those younger than 12 years (Table 24). Using these guides as a foundation, a practitioner can use the stepwise approach to manage a patient’s asthma efficiently with selected long-term controller medications (Figure 15).
According to asthma guidelines from the National Heart, Lung, and Blood Institute (NHLBI), the new focus in monitoring asthma control is based on distinguishing between asthma severity and asthma control:
- Asthma severity is defined as the “intrinsic intensity of the disease process.”4Practitioners should evaluate asthma severity before initiating therapy.
- Asthma control is defined as the “degree to which the manifestations of asthma…are minimized by therapeutic interventions and the goals of therapy are met.”4
Practitioners should then assess and monitor asthma control in order to adjust therapy based on the stepwise approach.
Within severity and control, the NHLBI focuses on 2 key domains: impairment and risk4:
- Impairment is the “frequency and intensity of symptoms and functional limitations the patient is experiencing currently or had recently experienced.”
- Risk is the “likelihood of either asthma exacerbations, progressive decline in lung function (or, for children, reduced lung growth), or risk of adverse effects from medication.”
Based on symptom severity and control, including the domains of risk and impairment, a practitioner can either step up or step down when adjusting a patient’s daily long-term controller medication. This updated stepwise approach now includes age-specific guidelines (ages 0-4, 5-11, and 12+) to aid with a more focused treatment plan. Several classes of medication are included in the stepwise approach used to manage asthma control.
Inhaled Corticosteroids
Inhaled corticosteroids (ICSs) are the mainstay long-term medication for asthma control. Results of recent studies have shown that consistent use of ICSs will improve asthma symptoms more so than with any other long-term controller medication available for patients of any age.2ICSs work by inhibiting inflammatory cytokines, thereby decreasing inflammation.6This prevents and reduces airway swelling and reduces mucus in the lungs.7Experts advise that ICSs are the first-line choice for controlling asthma because they decrease the number of asthma exacerbations, symptom frequency, and oral steroid use.2Examples of ICSs include beclomethasone (QVAR), budesonide (Pulmicort), ciclesonide (Alvesco), flunisolide (Aerospan), fluticasone (Flovent), and mometasone furoate (Asmanex Twisthaler).8The only ICS that is classified as pregnancy category B is budesonide.6ICSs can be used long term as a safe and effective treatment for persistent asthma in patients of all ages.5
Leukotriene Receptor Antagonists
Leukotrienes are chemicals in the body that are released when a patient breathes in an allergen such as pollen. When leukotrienes are released into the body, swelling in the lungs and vasoconstriction of the airway can occur, potentially precipitating asthma symptoms.6Blocking the leukotriene response terminates this reaction in the body. The 2 available leukotriene receptor antagonists are montelukast (Singular) and zafirlukast (Accolate). Montelukast is given once a day and can be given to children older than 12 months. Zafirlukast is given twice daily and can be given to children older than 7 years.2Both are rated as pregnancy category B and are suitable for managing mild persistent asthma. Leukotriene receptor antagonists have a high rate of compliance because of their ease of use, and they also provide great control of asthma symptoms.2
Long-Acting Beta2-Agonists
Long-acting beta2-agonists (LABAs) include salmeterol (Serevent) and formoterol (Foradil). These medications are bronchodilators that selectively stimulate the beta2adrenergic receptors, allowing the airways to vasodilate by relaxing the smooth muscles around them.7Because LABAs are specific to beta2adrenergic receptors, associated rates of tremor and tachycardia are low.2LABAs should never be used as monotherapy for asthma control. As a result of increased asthma exacerbations and a rise in related deaths when LABAs were used alone, the FDA reviewed these medications and determined that they should be used only as part of combination therapy with an ICS.2LABAs now require a black-box warning and are categorized as pregnancy class C. Pregnant patients should be advised by their practitioner about whether the benefit of taking a LABA outweighs the risk. LABAs should be considered for combination therapy when ICSs alone are not improving asthma symptoms.
Cromolyn Sodium
Cromolyn sodium is an inhaled nonsteroidal medication that stabilizes mast cells to prevent the airways from swelling when they come in contact with an asthma trigger.6,7Although cromolyn sodium is used as an alternative medication option for asthma control, it is not an ideal medication. Newer medications are available that can better control asthma.
Immunomodulators
Omalizumab (Xolair) is an immunomodulator medication that is used as additive therapy for patients aged 12 years and older who have severe persistent asthma and demonstrated immediate hypersensitivity to inhaled allergens.2It prevents binding of immunoglobulin E to mast cells and basophils, thereby inhibiting the body from reacting to allergic triggers.6Omalizumab is administered as a subcutaneous injection every 2 to 4 weeks. At a cost of approximately $1048 for a single injection, however, the medication is expensive.6In addition, it is advised that this medication be administered and monitored by an asthma specialist because of the black-box warning regarding the high risk for anaphylaxis.
Methylxanthines
Theophylline is a methylxanthine that is used as an alternative medication in patients with asthma, most effectively in combination with an ICS. Theophylline serum levels need to be monitored in patients taking this methylxanthine because toxicity can occur.2Although theophylline is still used in some clinical situations, it is no longer the preferred asthma control treatment because patients do not often comply with monitoring serum theophylline levels. Newer, more effective medications are available.
Combination Therapy
Combination therapy in patients with asthma consists of an ICS combined with an LABA. Examples include budesonide/formoterol (Symbicort), fluticasone/salmeterol (Advair), and mometasone/formoterol (Dulera).6Combination therapy is convenient for patients because it decreases the need for 2 separate inhalers. It is also safer for patients because the medications are already combined, decreasing potential side effects and lowering the incidence of black-box warning side effects with LABAs. Results from various studies have shown outstanding asthma control with combination therapy for moderate persistent asthma in patients aged 12 years and older.2
Oral Steroids
Oral steroid treatment is recommended not only for moderate to severe asthma exacerbations, but also for step 6 in the stepwise approach to asthma management (Figure 15). Dosing for oral steroid treatment is 1­ mg/kg/day to 2 mg/kg/day for 3 to 10 days in children, or 40 mg/kg/day to 60 mg/day in 1 or 2 divided doses for 5 to 10 days in adults. Tapering is not necessary because of the short course of treatment.2
Important Controller Medication Interactions
When taking a patient’s history, practitioners should ask several questions regarding current medications because many of them can interact with long-term asthma controller medications. In particular, any beta-blocker or angiotensin-converting enzyme (ACE) inhibitor use should be evaluated. A risk for bronchospasm is associated with nonselective beta-blockers due to the involvement of beta1and beta2receptors. Beta2-receptor blockers cause bronchoconstriction and should be used with caution in patients with asthma. Additionally, ACE inhibitors should be used with caution because many patients present with coughing as a common side effect. Other medications that can cause bronchospasm in an older asthmatic patient and should be given with caution include nonsteroidal anti-inflammatory drugs and cholinergic agents.5These and many other factors should be considered when prescribing medications for patients with asthma. Practitioners should always weigh benefits and risks when prescribing long-term control medications because treatment is ultimately about deciding what is best for the patient.
Other Ways to Optimize Asthma Control
Although long-term controller medications are the foundation for asthma control, it is imperative for patient education to be provided, as well. In order to optimize asthma control, patients need to be educated about an asthma action plan, peak flow use, severity of dyspnea, the need for follow-up, and recommended vaccines.
To effectively use an asthma action plan, a patient must be educated on how to perform a PFM. Peak flow averages are based on gender, age, and height or a patient’s “personal best” effort,5which is determined when the patient is asymptomatic and his or her asthma is under control. With a combination of a patient’s averages and personal best, an asthma action plan can be created (Figure 25).

The asthma action plan used most commonly involves the traffic light model of green, yellow, and red zones. Using this model, patients can identify that they are within the green zone when they are not having any symptoms and their PFM is 80% or greater of their personal best. Meanwhile, patients in the yellow zone have some symptoms and a PFM that is 50% to 80% of their personal best, while those in the red zone are severely short of breath with critical asthma symptoms and a PFM less than 50% of their personal best.5Patient self-monitoring with PFMs and symptoms using an asthma action plan allows for superior asthma control and improved patient outcomes.
It is important for practitioners to follow up and recommend vaccines for patients to better control their asthma, as this follow-up can produce better health outcomes. It is recommended that patients be reevaluated 2 to 6 weeks after the introduction of a new medication.3Patients with controlled persistent asthma should have scheduled biannual follow-up visits.2Asthma control can be stepped down at this time in patients who are very well controlled for at least 3 months at their current step using the stepwise approach. During these visits, practitioners can also encourage patients to obtain recommended vaccines.
The CDC recommends that asthma patients receive the influenza vaccine annually. It also recommends that patients aged 19 to 64 years, and all adults aged 65 years and older, receive the pneumococcal polysaccharide vaccine.9
Conclusion
Improved asthma control requires a foundation of long-term controller medications alongside patient education and self-monitoring. When the pieces of asthma control are all put into place, practitioners can beyond a doubt provide exceptional care for their patients.
Tiffany Budzinski is a board-certified family nurse practitioner specializing in retail health. She currently works for CVS MinuteClinic. She earned her bachelor of science in nursing degree at University of Illinois in Chicago and her master’s of science in nursing at Saint Xavier University in Evergreen Park, Illinois.
References
- Slejko J, Ghushchyan VH, Sucher B, et al. Asthma control in the United States, 2008-2010: indicators of poor asthma control.J Allergy Clin Immunol. 2014;133(6):1579-1587. doi: 10.1016/j.jaci.2013.10.028.
- Elward KS, Pollart SM. Medical therapy for asthma: updates from the NAEPP guidelines.Am Fam Physician. 2010;82(10):1242-1251.
- Pollart SM, Elward KS. Overview of changes to asthma guidelines: diagnosis and screening.Am Fam Physician. 2009;79(9):761-767.
- Expert Report Panel 3: Guidelines for the Diagnosis and Management of Asthma.NIH Publication No. 07-4051. Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. nhlbi.nih.gov/files/docs/guidelines/asthgdln.pdf. Accessed March 6, 2016.
- Rance K, O’Laughlen M. Managing asthma in older adults.J Nurse Pract. 2014;10(1):1-9.
- Epocrates. Drugs: asthma/pulmonary. https://online.epocrates.com/drugs/. Accessed February 19, 2016.
- Asthma and Allergy Foundation of America. Asthma treatment. aafa.org/page/asthma-treatment.aspx. Reviewed September 2015. Accessed March 8, 2016.
- Epocrates. Inhaled corticosteroid dose comparison. https://online.epocrates.com/tables/3249/Inhaled-Corticosteroid-Dose-Comparison-Adult. Accessed March 7, 2016.
- Centers for Disease Control and Prevention. Asthma. cdc.gov/asthma/. Updated March 7, 2016. Accessed March 9, 2016.
Asthma action plans with peak flow ranges are vital in order to improve patient outcomes. Patients with asthma frequently misperceive the severity of dyspnea because they have a longstanding history of shortness of breath which makes it difficult for them to realize when their asthma symptoms are worsening.5Results from a recent study show that only 19.1% of asthma patients reported having a peak flowmeter at home,1which further proves the need for education with an asthma action plan and use of peak flowmeter measurements (PFMs).

Knock Out Aches and Pains From Cold
October 30th 2019The symptoms associated with colds, most commonly congestion, coughing, sneezing, and sore throats, are the body's response when a virus exerts its effects on the immune system. Cold symptoms peak at about 1 to 2 days and last 7 to 10 days but can last up to 3 weeks.
COPD: Should a Clinician Treat or Refer?
October 27th 2019The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines the condition as follows: “COPD is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.â€
Diabetic Ketoacidosis Is Preventable With Proper Treatment
October 24th 2019Cancer, diabetes, and heart disease account for a large portion of the $3.3 trillion annual US health care expenditures. In fact, 90% of these expenditures are due to chronic conditions. About 23 million people in the United States have diabetes, 7 million have undiagnosed diabetes, and 83 million have prediabetes.
What Are the Latest Influenza Vaccine Recommendations?
October 21st 2019Clinicians should recommend routine yearly influenza vaccinations for everyone 6 months or older who has no contraindications for the 2019-2020 influenza season starting at the end of October, according to the Advisory Committee on Immunization Practices.
What Is the Best Way to Treat Pharyngitis?
October 18th 2019There are many different causes of throat discomfort, but patients commonly associate a sore throat with an infection and may think that they need antibiotics. This unfortunately leads to unnecessary antibiotic prescribing when clinicians do not apply evidence-based practice.
