Tuberculosis Screening
Annual screening for tuberculosis has become a familiar process for many. Anyone working or volunteering in the health care industry or who may come in contact with high-risk populations has a risk of exposure and may be required to have periodic testing.
Annual screening for tuberculosis (TB) has become a familiar process for many. Anyone working or volunteering in the health care industry or who may come in contact with high-risk populations has a risk of exposure and may be required to have periodic testing. Even college students are often expected to receive TB screening prior to beginning classes.
The history of TB can be traced back to the Middle Ages.1Over time, it has been known by various names, including phthisis pulmonalis, Pott’s disease, scrofula, the white plague—based on the pallor seen among infected patients—and consumption because it appears to consume the patient, causing profound weight loss.1Archeolo- gists have found TB in relics from ancient Egypt, India, and China.1
TB develops after exposure to the organismmycobacterium tuberculosis(M. tb).2It is airborne, spreading from one patient to another when an infected patient sneezes, coughs, speaks, sings, or laughs.3Individuals in close proximity can breathe the TB bacteria into their lungs, and this exposure may lead directly to an active TB infection. Some individuals who are exposed and breathe in the bacteria do not immediately develop the disease, however. While the bacteria live in their body, they do not feel sick, do not have symptoms, and cannot spread the disease to others; this is called latent TB infection (LTBI).3
Latent Tuberculosis
One-third of the world's population is estimated to have LTBI, the persistent immune system response from exposure to the TB organism.4Over time, LTBI may develop into TB disease through the process of TB reactivation. The risk of reactivation in a healthy individual is estimated to be less than 10% and typically occurs within 5 years of becoming infected. Risk of reactivation is higher in children and in patients who have other predisposing factors, like HIV.4Reactivation of LTBI accounts for approximately 80% of TB cases.5
Tuberculosis Disease
Progression from LTBI to the disease of TB occurs only if the bacteria overcome the natural defense of our body’s immune system.2This progression can occur soon after infection, or it may happen later in response to a weakened immune system. Symptoms may include unexplained weight loss, loss of appetite, night sweats, fever, fatigue, and chills. A cough lasting longer than 3 weeks, hemoptysis, and chest pain are also frequently seen with the disease. Additional symptoms may be reported if the infection is affecting areas of the body other than the lungs.2
An increased risk of progression to TB from LTBI is seen in specific populations. This is especially a concern in HIV-infected individuals. According to the World Health Organization, in 2015, globally, 55% of patients with TB had documented HIV test results.6Efforts to control TB around the world have focused heavily on detection and prevention of TB infection in this group. Additional factors that increase the risk of progression from LTBI to TB include individuals with chronic renal failure or those on hemodialysis; patients with diabetes, gastrectomy, jejunoileal bypass, organ transplant, and head or neck cancer7; and individuals who inject illegal drugs.8Even having a low body weight (more than 10% below ideal levels) increases the risk of progression from LTBI to active TB disease. Age can also be a factor. Elderly patients, babies, and young children 8 are considered at increased risk.
Screening
Testing for TB should focus on those who are at higher risk for becoming infected with the bacteria. The risk is greatest for individuals who have spent time with someone who has TB disease. This includes living or working in high-risk settings, such as correctional facilities, long-term care facilities, nursing homes, and homeless shelters.8Additionally, screening should be considered for anyone living among high concentrations of people, like refugee camps, mental health institutions, incoming college students from high incident countries, and military personal.9,10
Anyone working or volunteering in the health care system may come in contact with those who have the disease and should be screened, too.8Scheduled TB screening of health care workers is an important part of an infection control plan and is required by law in some states. Concerns of transmission have also prompted screening of people from countries where TB is more common. This includes most countries in Latin America, Africa, Asia, and Eastern Europe.10Screening should also be considered for those who have any of the known risk factors for TB, including HIV or a weakened immune system.4
Two screening test options are available to determine if a person is infected with the TB bacteria: the tuberculin skin test (TST) and the TB blood test.11Development of the TST began with Clemens von Pirquet, an Austrian-born scientist and physician who began using a small amount of isolated TB bacteria and scratching it into the skin to observe for a reaction. Expanding on the work of von Pirquet, Charles Mantoux developed the TST, which has remained, the standardized screening test for TB since 1907. The TST has also been called the Pirquet skin test, the Mantoux skin test, and the tuberculin sensitivity test.12
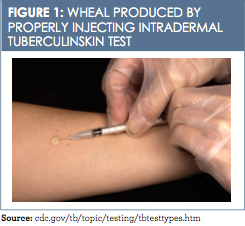
TST can be administered the same day as live virus vaccinations. If not completed on the same day, the testing should be completed 4 to 6 weeks after live virus vaccine administration.13The standardized procedure for TST includes using a 27-gauge tuberculin syringe, bevel facing upward, to inject 0.1 ml of purified protein derivative on the inner aspect of the forearm. This intradermal injection, when placed correctly, produces a wheal (a pale ele- vation of the skin) 6 to 10 mm in diameter (Figure 1).13 The test must be read between 48 and 72 hours after placement; if not read within this timeframe, the test must be repeated. The second test can be given immediately because there is no contraindication to repeat testing.13
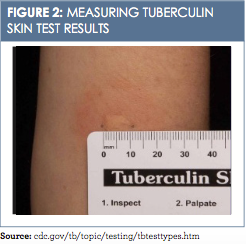
Reading the TST and interrupting the results include measuring the induration and assessing the patient’s risk of TB infection. The reaction or induration should be measured in millimeters. Induration is defined as the hardened, palpable, raised area only and should not include measuring of erythema (redness) (Figure 2).13A measurement of 5 mm or more is read as a positive TST in the following populations:
• HIV-infected patients
• Those with recent contact with an individual who has TB
• Patients who have had organ transplants
•Those with known immunosuppression (eg, taking long-term, high-dose steroids)
An induration of 10 mm or more is read as a positive TST in these populations:
•Migrants from high-prevalence countries within the last 5 years
• Injection-drug users
• Individuals who live or work in high-risk settings
• Patients with a diagnosis of conditions from high-risk categories
• Children younger than 4 years
• Infants, children, and adolescents who have had exposure to adults from high-risk categories
All reactions with an induration of 15 mm or more are considered positive, even if no risk factors exist.13
Some false-positive TST reactions have been documented. These reactions may be attributed to individuals’ being infected with nontuberculosis mycobacteria, having a history of bacillus Calmette-Guérin (BCG) vaccination, or receiving improper TST placement or interpretation.13
Individuals infected with TB may not react to the TST and have a false-negative reaction. Possible explanations for false-negative results include recent TB infection (within the first 10 weeks of exposure), remote past history of TB infection, recent live virus vaccinations, or improper TST placement or interpretation.13
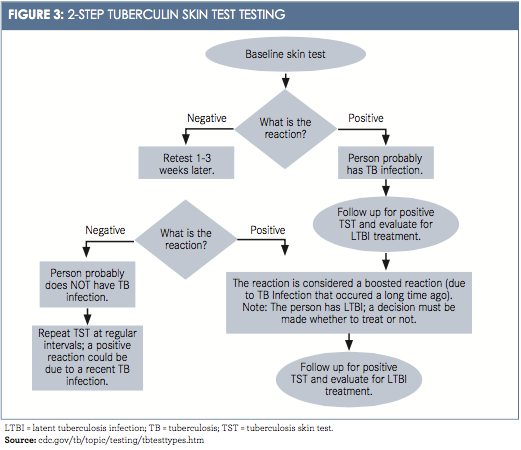
A 2-step TST is now commonly used for health care workers. After the ini- tial TST is negative, a second TST is administered between 1 and 3 weeks later. The first TST may boost or stimulate a reaction to the second TST. This more comprehensive testing could reveal LTBI from exposures years earlier. These 2-step tests are typically used as initial or baseline screening only, mostly for new hires. Thereafter, routine annual TST screening of health care employees is recommended (Figure 3).14Interferon-Gamma Release Assay (IGRA) is the blood test used to detect TB bacteria.
Currently, 2 approved versions of the test are available in the United States. Lab protocols for collection and handling requirements of the blood should be reviewed prior to conducting this test. In the lab, the blood is used to test the patient’s immune system reaction to the TB bacteria.3A positive IGRA test indicates the patient has LTBI or TB disease. A negative IGRA test indicates that the patient’s blood did not react to the test and infection with LTBI or TB disease is very unlikely.3
Deciding which test to use, TST or IGRA, can depend upon reasons for testing, test availability, and cost. No contraindication exists for using the IGRA test, and use of this test is preferred if the patient has received the BCG vaccine.13This vaccine is not recommended in the United States, but it is given in some high-risk countries at birth to prevent TB infection.7Results of a TST may be positive if the vaccine has been previously given.7The only contraindication to the TST is a severe reaction to a previous TST. Severe reactions include blistering, ulcerations, necrosis, and anaphylactic shock. TST is safe for infants, children, and pregnant women.13Simultaneous testing with both TST and IGRA is not recommended.3Neither test can discriminate between LTBI, TB disease, and treated infections.4
Neither negative TST nor IGRA indicates that the person did not react to the test and is unlikely to have TB. Positive TST or IGRA results indicate the patient is infected with TB bacteria and further evaluation is needed.3A complete medical history and chest radiography with anterior, posterior, and lateral views are the usual initial assessments.3Chest x-ray has a high sensitivity and is well suited for early detection of TB.4Next, sputum samples are examined for acid-fast bacilli and cultured for mycobacteria.15Additional lab tests may also be ordered. After testing, if the patient is found not to have TB disease, but has TB bacteria present in the body, the diagnosis of LTBI is then made.3
Current treatment options for LTBI can reduce the risk of developing active TB by as much as 90%.5A concern related to these treatments is the risk of hepatotoxicity. Close clinical and laboratory monitoring is required with the use of these therapies. Decisions about the treatment of LTBI are usually based on the likelihood of developing TB disease considering overall health and risk factors.3Positive diagnosis of TB disease requires treatment with antituberculosis therapy, which consists of several medications, for a period of 6 to 12 months.16The CDC and Infectious Diseases Society of America have comprehensive treatment guidelines available for treatment planning.16These guidelines urge beginning treatment as quickly as possible to decrease the likelihood of additional exposures. Common medications used for treatment include ethambutol, isoniazid, pyrazinamide, and rifampin. Close monitoring of patients receiving treatment is essential to assess for complications, such as drug— drug interactions.16
Many new TB cases are diagnosed in marginalized groups, where access to treatment can be more difficult, making adherence a greater issue.17However, adherence to the medication regimen is essential for a cure. Drug-resistant TB developed when patients did not take their medications as prescribed or stopped their medications too soon. Therefore, in some communities, health department staff meet regularly with patients who are receiving medications for the treatment of TB disease just to observe the patient taking their medications. This process of direct observational therapy helps prevent drug-resistant strains of TB.16
Conclusion
Since 1993, the number of new TB cases in the United States has continued to decline. At present, incidence rates are the lowest ever seen, with only 3 cases per 100,000 individuals. Still, 9412 new TB cases were reported last year.18There is still work to be done: at the current rate of decline, achieving elimination of TB will take another 70 years.5 In addition, because more than 13 million individuals in the United States are infected with LTBI, yet elimination of TB is dependent on preventing new cases. This is best achieved by screening to detect LTBI and preventing reactivation.4
Premature death and avoidable adverse experiences result from TB, a treatable disease with a cure. Strategies to screen high-risk groups and individuals must continue. Following up with newly diagnosed cases of TB quickly can decrease new exposures and increase the chances of cure.
Dr. Lisa Bridwell Robinson, DNP, CCRN, CNE, NP-C, is a family nurse practitioner and certified nurse educator who practices in a retail clinic and as a worksite health coach. Dr. Robinson also serves as a faculty member for graduate nursing students in both masters and doctoral programs of study.
References
1. Mandal A. History of tuberculosis. Medical Life Sciences website. news-medical.net/health/History-of-Tuberculosis.aspx. Updated February 3, 2014. Accessed September 28, 2016.
2. TB elimination: the difference between latent TB infection and TB Disease. CDC website. cdc.gov/tb/publications/factsheets/general/ltbiandactivetb.htm. Published November 2011. Accessed September 9, 2016.
3. Testing for tuberculosis. CDC website. cdc.gov/tb/publications/factsheets/testing/tb_testing.htm. Updated September 1, 2016. Accessed October 3, 2016.
4. Latent tuberculosis infection: Scaling up programmatic management of LTBI, a critical action to achieve the WHO End TB Strategy targets. World Health Organization website. who.int/tb/publications/ltbi_factsheet_161014.pdf. Accessed September 28, 2016.
5. Mancuso J, Diffenderfer J, Ghassemieh B, Horne D, Kao T. The prevalence of latent tuberculosis infection in the United States.Am J Respir Crit Care Med.2016;194(4):501-508. doi: 10.1164/rccm.201508-1683OC.
6. Global tuberculosis report 2015. World Health Organization website. who.int/tb/publications/factsheet_global.pdf. Accessed November 15, 2016.
7. TB elimination: targeted tuberculosis testing and interpreting tuberculin skin test results. CDC website. cdc.gov/tb/publications/factsheets/testing/skintestresults.pdf. Published December 2011. Accessed November 15, 2016.
8. Tuberculosis (TB): who should be tested. CDC website. cdc.gov/tb/topic/testing/whobetested.htm. Updated September 8, 2016. Accessed November 15, 2016.
9. Improving early detection of active TB through systematic screening. World Health Organization website. who.int/tb/publications/tbscreening_factsheet.pdf?ua=1. Published 2013. Accessed November 15, 2016.
10. American College Health Association Guidelines. Tuberculosis screening and targeted testing of college and university students.J Am Coll Health.2011;59(7):670-677. doi: 10.1080/07448481.2011.596733.
11. Linas BP, Wong AY, Freedberb KA, Horsburgh CR Jr. Priorities for screening and treatment of latent tuberculosis infection in the United States.Am J Respir Crit Care Med.2011;184(5):590-601. doi: 10.1164/rccm.201101-0181OC.
12. Historical Perspectives Centennial: Koch’s discovery of thetubercle bacillus. CDC website. cdc.gov/mmwr/preview/mmwrhtml/00000222.htm. Published March 19, 1982. Accessed November 15, 2016.
13. TB elimination: tuberculin skin testing. CDC website. cdc.gov/tb/publications/factsheets/testing/skintesting.pdf. Published October 2011. Accessed November 15, 2016.
14. Tuberculosis (TB): testing health care workers. CDC website.
cdc.gov/tb/topic/testing/healthcareworkers.htm. Updated April 15, 2016. Accessed November 15, 2016.
15. Triasih R, Robertson CF, Duke T, Graham SM. A prospective evaluation of the symptom- based screening approach to the management of children who are contacts of tuberculosis cases.Clin Infect Dis. 2015;60(1):12-18. doi: 10.1093/cid/ciu748.
16. Nahid P, Dorman SE, Alipanah N, et al. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Disease Society of American Clinical Practice Guidelines: treatment of drug susceptible tuberculosis.Clin Infect Dis.2016;63(7):e147-e195. doi: 10.1093/cid/ciw376.
17. Whalen C. The replacement principle of tuberculosis: why prevention matters.Am J Respir Crit Care Med.2016;194(4):400-401.doi: 10.1164/rccm.201603-0439ED.
18. Tuberculosis (TB): fact sheet: trends in tuberculosis, 2014. CDC website. cdc.gov/tb/publications/factsheets/statistics/tbtrends.htm. Accessed November 8, 2016.

Knock Out Aches and Pains From Cold
October 30th 2019The symptoms associated with colds, most commonly congestion, coughing, sneezing, and sore throats, are the body's response when a virus exerts its effects on the immune system. Cold symptoms peak at about 1 to 2 days and last 7 to 10 days but can last up to 3 weeks.
COPD: Should a Clinician Treat or Refer?
October 27th 2019The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines the condition as follows: “COPD is a common, preventable, and treatable disease that is characterized by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases.â€
Diabetic Ketoacidosis Is Preventable With Proper Treatment
October 24th 2019Cancer, diabetes, and heart disease account for a large portion of the $3.3 trillion annual US health care expenditures. In fact, 90% of these expenditures are due to chronic conditions. About 23 million people in the United States have diabetes, 7 million have undiagnosed diabetes, and 83 million have prediabetes.
What Are the Latest Influenza Vaccine Recommendations?
October 21st 2019Clinicians should recommend routine yearly influenza vaccinations for everyone 6 months or older who has no contraindications for the 2019-2020 influenza season starting at the end of October, according to the Advisory Committee on Immunization Practices.
What Is the Best Way to Treat Pharyngitis?
October 18th 2019There are many different causes of throat discomfort, but patients commonly associate a sore throat with an infection and may think that they need antibiotics. This unfortunately leads to unnecessary antibiotic prescribing when clinicians do not apply evidence-based practice.
